Hernia Mesh Complications Spark Lawsuits Against Atrium and Ethicon
Injured?
Atrium C-QUR, Ethicon Physiomesh Hernia Mesh Allegedly Cause Severe Intestinal Complications
Our attorneys are currently investigating claims that certain hernia surgical mesh implants made by medical manufacturing companies Atrium Medical and Johnson & Johnson subsidiary Ethicon are defective and have caused serious injuries to hernia surgery patients.
If you have received surgical mesh during a recent hernia repair surgery, you could be at risk of adverse medical effects and complications including bowel obstruction, mesh adhesion to tissue, hernia recurrence, and more. Contact us to learn if you could be eligible for compensation for suffering from these serious injuries.
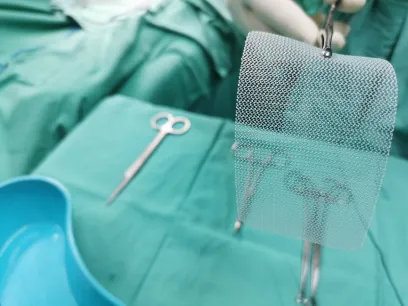
What is a Hernia Mesh and Why Are People Reporting Problems with It?
Patients harmed by a defective hernia mesh often must undergo repeated surgeries to remove the device.
A hernia mesh is a medical device used to support weakened or damaged tissue following a surgery to repair a hernia.
Both Atrium’s C-QUR and Ethicon’s Physiomesh hernia mesh implants have reportedly caused serious injuries in patients who underwent hernia surgery — ranging from pain and infection to adhesion to tissue and bowel obstruction. There are currently about 20 Physiomesh and 24 C-QUR hernia mesh lawsuits filed in federal court.
Ethicon’s Physiomesh Lawsuits Allege the Product Caused Permanent Injuries
Doctors implanted a Florida woman with an Ethicon Physiomesh flexible composite mesh during hernia repair surgery and she suffered permanent injuries as a result, according to the lawsuit Quinn v Ethicon, Inc. filed in the U.S. District Court for the Middle District of Florida.
The suit claims that Physiomesh as a product is defective, prone to becoming embedded in human tissue over time, and is “inappropriately designed and engineered for use in hernia repair,” creating an unreasonable risk of injury to patients.
Atrium C-QUR Mesh Lawsuits Allege the Mesh’s Gel Coating Is Defective
One Georgia woman suffered seroma (a buildup of fluids in the body) and nerve damage to the abdomen after receiving a defective C-QUR mesh implant, according to the lawsuit Bryant v. Atrium, filed in the U.S. District Court for the Middle District of Georgia. As a result of the mesh and the consequent removal of the implant, the plaintiff allegedly missed six weeks of work and develop scar tissue buildup in her abdomen.
The suit claims the C-QUR Mesh’s Omega 3 gel coating made from fish oil is a major defect in the design of the implant, because it can cause the implant to curl and deform over time, leading to serious inflammation and injuries.
Defective Hernia Mesh Complications

The known complications associated with defective hernia mesh products include:
- Adhesion to Tissue
- Bowel Obstruction
- Bowel Perforation
- Hernia Recurrence
- Mesh Migration
- Mesh Shrinkage
- Seroma
- Pain
- Infection
Additionally, patients harmed by a defective hernia mesh often must undergo repeated surgeries to remove the device.
Have Any Hernia Mesh Product Recalls Been Issued?
Yes. In 2013, the FDA issued a Class II recall on one of Atrium’s C-QUR hernia mesh products, after finding that the product’s fish oil coating could peel off and stick to the inside of its package when exposed to excessive humidity. This is the same fish oil coating that is alleged to cause the mesh to curl and deform in the body.
Ethicon voluntarily recalled certain models of the Physiomesh in 2016 after studies revealed high hernia recurrence rates in patients with that particular hernia mesh implanted, according to medical device business journal MassDevice.
Am I Eligible for a Hernia Mesh Lawsuit?
If you have undergone hernia repair surgery and received a hernia mesh implant, our attorneys want to hear from you, even if you’re unsure what type of hernia mesh you have. You may qualify for a hernia mesh lawsuit if you meet the following criteria:
- You underwent a laparoscopic hernia repair surgery that used hernia surgical mesh.
- The surgery took place on or after Jan. 1, 2008.
- You had or have scheduled hernia revision surgery.
Be sure to record any adverse symptoms you may have experienced after your hernia surgery. This information will be important if you choose to participate in a lawsuit.
What Can I Collect in a Lawsuit?
By participating in a lawsuit against Atrium and Ethicon, you may be able to receive compensation for past and future medical expenses, loss of income, and other damages. Your participation will also serve to hold these medical manufacturers responsible for putting defective products out on the market.
To learn more about joining a lawsuit, contact Morgan & Morgan today. We’re here to help you.

We've got your back
Injured?
Not sure what to do next?
We'll guide you through everything you need to know.
